Question
Calculate the velocity of electron in the first Bohr orbit of hydrogen atom. Given that
Bohr radius = 0.529 Å,
Planck’s constant, h = 6.626 × 10–34 Js,
mass of electron = 9.11 × 10–31 kg and
1 J = 1 kg m2s–2.
SIMILAR QUESTIONS
Q1
The Vividh Bharati station of All India Radio, Delhi broadcasts at a frequency of 1368kHz (kilohertz). Calculate the wavelength of the electromagnetic radiation emitted by the transmitter. Which part of the electromagnetic spectrum does it belong to?
Q2
The wavelength range of the visible spectrum extends from violet (400 nm) to red (750 nm). Express these wavelengths in frequencies (Hz) (1 nm = 10–9 m)
Q3
Calculate the frequency and energy of a photon of radiation having wavelength 6000 Å.
Q4
Calculate the energy of a mole of photons of radiations whose frequency is 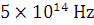 ?
?
Q5
A 100 watt butt bulb emits monochromatic length of wavelength 400 nm. Calculate the number of photons emitted per second by the bulb.
Q6
Calculate the kinetic energy of the electron eject when yellow light of frequency 5.2 × 1014 sec–1 falls on the surface of potassium metal. Threshold frequency of potassium is 5 × 1014 sec–1.
Q7
The threshold frequency v0 for a metal is 7.0 × 1014 s–1. Calculate the kinetic energy of an electron emitted when radiation of frequency v = 1.0 × 1015 s–1 hits this metal.
Q8
Calculate the wavelength of the spectral line obtained in the spectrum of Li2+ ion when the transition takes place between two levels whose sum is 4 and the difference is 2.
Q9
Calculate the wavelength of the radiation emitted when an electron in a hydrogen atom undergoes a transition from 4th energy level to the 2nd energy level. In which part of the electromagnetic spectrum does this line lie?
Q10
Calculate ionization energy of the hydrogen atom.
